Biotechnology & Drug Development
The strict and continual application of aseptic processing, environmental controls and monitoring are key to the approval and success of your final drug or product as a leading solution. With Bioquell’s automated, validated biodecontamination, you have confidence in your bioburden elimination of your workspace and enhancements for sterility testing.
APPLICATIONS
Batch Production and Changeover
Vaccine Development
Equipment Decontamination
Pass-Through and Material Airlock Decontamination

Batch Production and Changeover
Your facility needs effective biodecontamination solutions to eradicate contaminants between production batches and changeovers. Our unique Hydrogen Peroxide Vapor technology will reset your environmental bioburden to zero, eliminating the risk of cross-contamination or product bio-tainting.
SYSTEMS AND SERVICES
Bioquell’s biotechnology and drug development decontamination provides vital total kill while saving time and costs, increasing production and achieving compliance. Whether you are operating our equipment yourself or relying on our experts for decontamination, Bioquell has the solution for your processes.
Discover more about our systems and services for your biotechnology and drug development work:
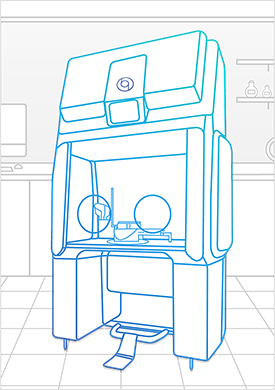
Bioquell Qube
The Bioquell Qube is a configurable isolator integrated with Bioquell’s Hydrogen Peroxide Vapor technology. From its unique design to rapid cycle times, the Bioquell Qube ensures your aseptic workspace needs are met for a safe and productive working environment.
Learn MoreBioquell ProteQ
The latest achievement from Bioquell, the Bioquell ProteQ offers a modular approach to room and zone decontamination. Enhanced distribution capabilities, networking options, wireless technology and advanced aeration capabilities make this system, the ideal solution for contamination elimination in the smallest labs to the largest production areas.
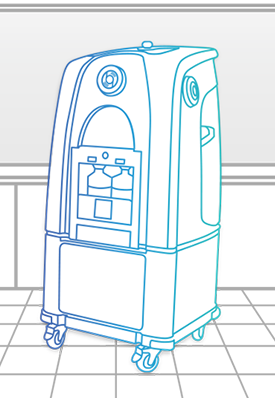
Bioquell L-4
The Bioquell L-4 is a mobile Hydrogen Peroxide Vapor generator that can be connected to enclosures and equipment in your facility or used for room/zone decontamination.
Learn MoreBioquell SeQure
The Bioquell SeQure is the fixed and compact wall-mounted biodecontamination system that eradicates the bioburden of microorganisms and other contaminants threatening your research and production facility.
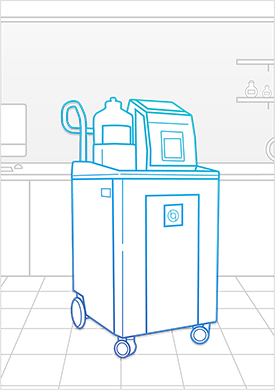
Learn MoreBioquell IG-2
The Bioquell IG-2 is a fixed system that becomes integral to your equipment and operating process by providing an integrated Hydrogen Peroxide Vapor solution for removing pathogens. We work closely with you to engineer the ideal fit for your required set-up and decontamination needs.
Learn MoreRapid Bio Decontamination Service
If you need decontamination but do not have the resources, time or equipment, Bioquell has your solution. From the smallest enclosure to a large building, our skilled personnel provide the customized treatment you require.
Learn MoreContact Us
To learn more about how Bioquell can fit your solution, please contact us.
The Americas
Ecolab Inc
702 Electronic Dr. Suite 200
Horsham, PA 19044
+1 215 682 0225
[email protected]